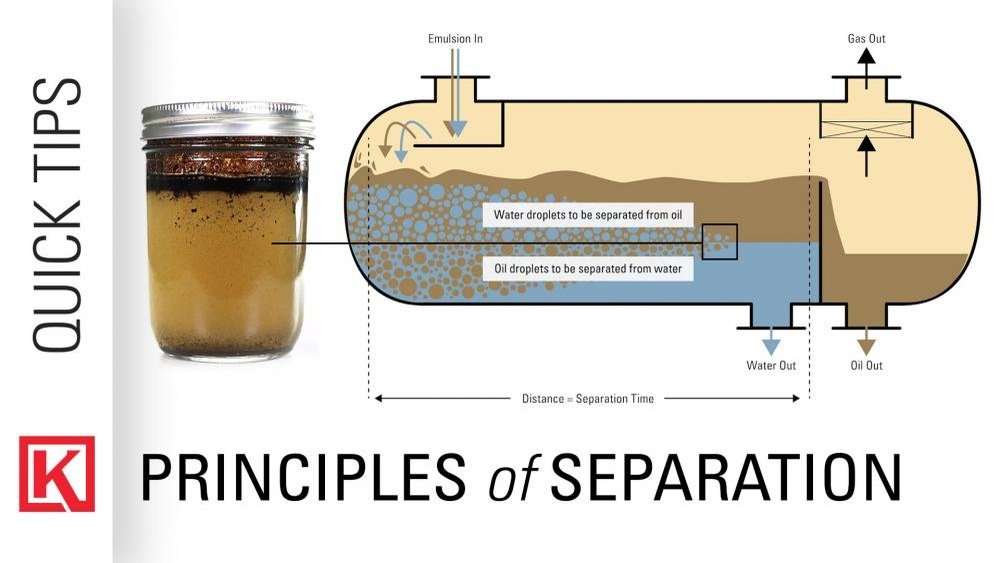
Have you ever mixed oil and water, only to watch them slowly drift apart after a while? That’s exactly what happens with emulsions—they start as smooth blends but often separate over time.
If you’ve wondered why your homemade salad dressing, cosmetic cream, or even a cooking sauce doesn’t stay perfectly mixed, you’re not alone. Understanding why emulsions separate can help you keep your mixtures stable and looking fresh longer. You’ll discover the surprising reasons behind emulsion separation and simple ways to prevent it.
Keep reading to unlock the secrets that will change how you handle emulsions for good.
Common Causes Of Emulsion Separation
Emulsions are mixtures of two liquids that usually do not mix, like oil and water. Over time, these mixtures can separate into layers. This separation happens because of certain common reasons. Understanding these causes helps in making better, longer-lasting emulsions.
Droplet Collision And Coalescence
Droplets inside an emulsion move and sometimes collide. When they hit each other, the thin film between droplets can break. This causes droplets to merge into bigger drops. Larger droplets separate faster, leading to visible layers.
Insufficient Emulsifiers
Emulsifiers are special molecules that hold droplets together. If there are not enough emulsifiers, the droplets cannot stay mixed. Without enough stabilizers, droplets join and separate easily. Proper emulsifier levels keep the mixture smooth and stable.
Ph And Temperature Effects
Changes in pH can alter emulsifier effectiveness. Extreme acidity or alkalinity weakens the protective layer around droplets. Temperature shifts also affect emulsion stability. High heat can cause droplets to move more and break apart.
Physical Instabilities: Creaming And Sedimentation
Creaming happens when lighter droplets rise to the top. Sedimentation occurs when heavier droplets sink. Both create layers in the mixture. These processes do not break droplets but cause uneven distribution. This leads to visible separation of the emulsion.
Role Of Emulsifiers In Stability
Emulsifiers play a crucial role in keeping emulsions stable. They work by helping oil and water mix and stay mixed. Without emulsifiers, these mixtures separate quickly into layers. Understanding how emulsifiers function helps explain why some emulsions last longer than others.
Types Of Emulsifiers
Emulsifiers come in many forms. Common types include natural emulsifiers like lecithin and egg yolk. Synthetic emulsifiers such as polysorbates and sodium stearoyl lactylate are also widely used. Each type works differently depending on the ingredients and desired stability.
How Emulsifiers Prevent Separation
Emulsifiers reduce surface tension between oil and water droplets. They form a protective layer around droplets, stopping them from merging. This layer prevents droplets from clumping and settling, which delays separation. Emulsifiers also help create smaller droplets for a smoother mix.
Limitations Of Emulsifiers
Emulsifiers cannot stop separation forever. Over time, droplets may still collide and break the protective layer. Changes in temperature or pH can weaken emulsifier effectiveness. Too much oil or water also overwhelms the emulsifier’s ability to keep droplets apart.
Chemical And Physical Factors Affecting Emulsions
Emulsions are mixtures of two liquids that normally do not mix, like oil and water. Their stability depends on many chemical and physical factors. These factors affect how well the droplets stay spread out. Over time, changes in these conditions cause emulsions to separate. Understanding these influences helps explain why emulsions break down.
Impact Of Ph Changes
pH levels can change the charge on the droplets’ surface. This affects how droplets repel or attract each other. If the pH shifts too far, the protective layer breaks down. Droplets then clump together and separate from the mixture. Many emulsifiers work best within a specific pH range. Outside that range, their ability to stabilize drops.
Temperature Fluctuations
Temperature changes affect the movement of droplets inside the emulsion. Higher temperatures increase droplet collisions, which can cause merging. Cooling may cause some parts to solidify, disrupting balance. Rapid temperature swings stress the emulsion and reduce stability. Keeping temperature steady helps maintain the emulsion’s uniform texture.
Mechanical Stress And Storage Conditions
Shaking, stirring, or vibration can break the delicate film around droplets. This mechanical stress leads to droplet merging and phase separation. Storage conditions like light exposure and air contact also affect emulsion life. Proper containers and gentle handling slow down separation. Emulsions stored in stable, cool places last longer.

Credit: www.researchgate.net
Advanced Techniques To Enhance Emulsion Stability
Emulsions can separate as droplets merge and settle over time. Advanced techniques help keep these mixtures stable longer. These methods improve the balance and strength of the emulsion.
Using the right ingredients and methods reduces droplet collision and breaking. This section explains key strategies to boost emulsion stability effectively.
Use Of Stabilizing Agents Like Polymers And Gums
Stabilizing agents such as polymers and gums increase the thickness of the liquid. This slows down droplet movement and merging. They form a protective layer around droplets to stop them from joining. Common gums include xanthan and guar gum. Polymers like carbomers help keep droplets evenly spread. These agents improve the emulsion’s texture and durability.
Optimizing Formulation Ratios
The ratio of oil to water affects emulsion stability. Too much oil overwhelms the emulsifiers, causing separation. A balanced ratio allows emulsifiers to coat all droplets. Adjusting the ratio ensures droplets remain small and stable. Testing different proportions helps find the best mix. Proper formulation reduces the chance of phase separation.
Processing Methods To Improve Stability
High-speed mixing and homogenization create smaller droplets. Smaller droplets resist merging better than larger ones. Proper temperature control during processing also matters. Cooling the emulsion slowly avoids stress that breaks droplets. Gentle but thorough mixing spreads ingredients evenly. These methods strengthen the emulsion’s structure and lifespan.
Troubleshooting And Expert Solutions
Troubleshooting emulsion separation requires careful analysis and expert approaches. Understanding the root causes helps prevent failures. Experts use specific methods to identify and fix instability. Practical steps extend the emulsion’s life and maintain its quality.
Identifying Instability Signs
Look for visible layers forming in the emulsion. Check for oil droplets clumping or floating to the top. Notice changes in texture or thickness. A sour or off smell may indicate chemical changes. Early detection prevents complete breakdown and product loss.
Adjusting Formulations
Balance the oil and water phases correctly. Increase or change emulsifiers to improve droplet coverage. Modify pH levels to keep emulsifiers stable. Add stabilizers like gums or polymers for stronger films. Small tweaks can restore emulsion uniformity and delay separation.
Practical Tips For Long-term Stability
Store emulsions at consistent, moderate temperatures. Avoid excessive shaking or agitation. Use fresh ingredients to reduce contamination risks. Incorporate antioxidants to prevent oxidation. Regularly test batches to ensure ongoing stability and quality.
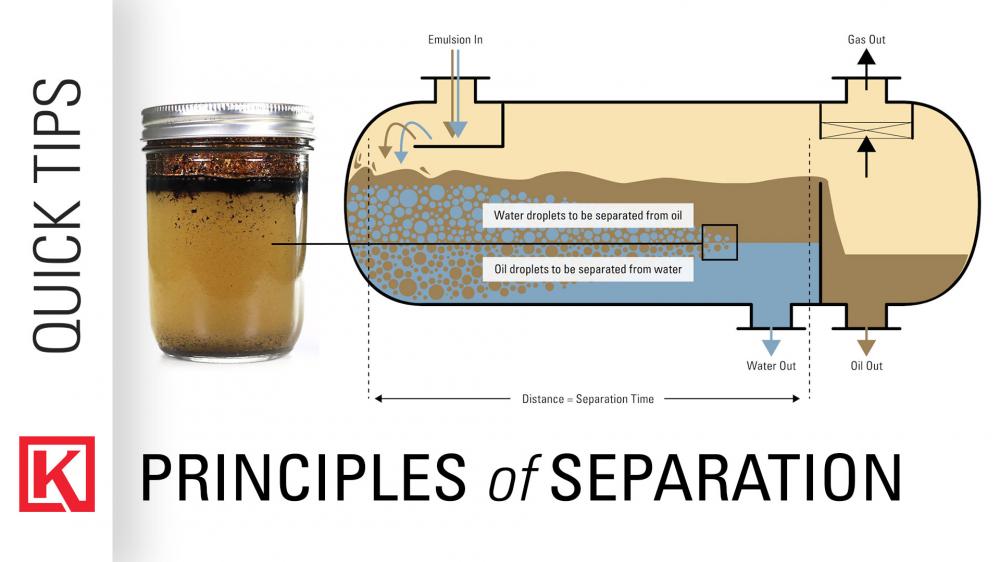
Credit: kimray.com
Credit: www.researchgate.net
Frequently Asked Questions
Why Did My Emulsion Separate?
Your emulsion separated because the emulsifiers couldn’t hold the oil droplets together. PH changes or droplet collisions also cause separation.
Why Do Emulsions Separate Over Time?
Emulsions separate as droplets merge, breaking the interfacial film between them. This causes larger droplets and phase separation over time. Changes in pH, insufficient emulsifiers, or physical processes like creaming also destabilize emulsions, leading to layer separation.
How To Prevent Emulsion From Separating?
Add proper emulsifiers and stabilizers to hold droplets evenly. Maintain consistent pH and avoid extreme temperatures. Stir gently to prevent droplet collision and rupture. Store emulsions in cool, stable conditions to enhance stability and prevent separation over time.
What Factors Cause A Break In Emulsion?
Factors causing emulsion break include excessive oil, insufficient emulsifiers, pH changes, droplet collisions, and instability like creaming or coalescence.
Why Do Emulsions Separate Over Time?
Emulsions separate because oil and water droplets merge, breaking the film that holds them together.
What Causes An Emulsion To Break Down?
Changes in temperature, pH, or droplet size can cause emulsions to become unstable and separate.
How Do Emulsifiers Prevent Emulsion Separation?
Emulsifiers create a barrier around droplets, keeping oil and water mixed and stopping them from separating.
Conclusion
Emulsions separate because tiny droplets join and grow bigger. This weakens the layer that keeps them mixed. Changes in temperature, pH, or ingredient amounts can speed up separation. Emulsifiers help keep droplets apart and stable longer. Still, over time, natural forces cause emulsions to break down.
Understanding these reasons helps you choose or store products better. Keeping emulsions stable requires careful formulation and handling. Simple steps can slow separation and extend shelf life. Recognizing why emulsions separate makes managing them easier every day.
 Skip to content
Skip to content